Engineered Polymer Membranes Offer Water Treatment Alternative

Dr. William Phillip. (Credit: Matt Cashore/University of Notre Dame, https://www.eurekalert.org/multimedia/pub/169728.php)
Earth’s freshwater reserves are scarce, and as the world population increases, that scarcity affects more people. In fact, about 1.9 billion people live with water scarcity, while another 2.1 billion live without safe drinking water management services—about half of the world, in total.
Increasing water scarcity and the pressure it exerts has prompted experts to search for novel, efficient methods for treating and reusing wastewater, and new ways to use brackish water as well as seawater. Nanotechnologies are at the forefront of this push for treatment alternatives.
A team of engineers from the University of Notre Dame and Purdue University has now advanced the study of self-assembled block polymer membranes, which can selectively remove contaminants, including salt, from various water sources. Typical polymer membranes suffer from a lack of specificity in the contaminants they remove; however, self-assembled block polymer membranes enable both uniform and customizable pore sizes for use in water treatment.
“The block polymers are made up of multiple segments (or blocks) of varying chemistry,” explains Notre Dame associate professor William Phillip, who is part of the Department of Chemical and Biomolecular Engineering and heads the Notre Dame Water Lab. “Some of these segments are hydrophilic and like water, and some are hydrophobic, and like oil, so they want to get away from each other just like oil and vinegar do in salad dressing. However, there are covalent bonds connecting the segments that prevent them from getting too far away from each other. Balancing these two constraints causes the block polymer to self-assemble into a variety of nanostructures once they are synthesized.”
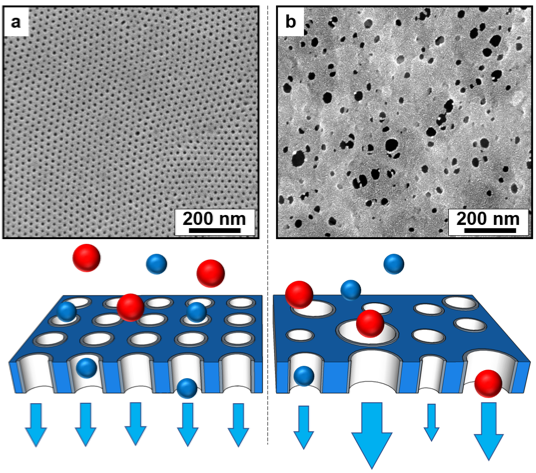
Figure 2. Scanning electron micrographs of (a) a self-assembled block polymer membrane and (b) a commercial membrane made using a standard non-solvent induced phase separation process. The highly-ordered nanostructure of the self-assembled membrane offers the potential for higher performance (i.e., higher throughput and more selective) separations relative to commercial membranes because the well-defined, narrow pore size distribution produces highly-selective filters, and generates a uniform flow distribution through each pore. The blue and red spheres represent solutes of varied size being filtered from solution. The width of the blue arrows is proportional to the volumetric flow through the associated pore. Solute selectivity and a uniform residence time for fluid flowing through the membrane pores are essential in the development of functional membranes for advanced applications. Adapted with permission from Reference 35. Copyright 2010 American Chemical Society. (Credit: Zhang et al., via communication with Notre Dame press office.)
By using self-assembled block polymer membranes, the team was able to show that the pore wall chemistry of the membranes they constructed could be controlled by engineering the nanostructure of the block polymer molecules. This will mean that scientists can isolate contaminants in water based on chemical identity, enabling decentralized wastewater reuse.
“We are beginning to identify the equilibrium nanostructures that lead to high-performance membranes, which allows us to design the block polymers for membrane applications,” details Dr. Phillip. “The process for transforming the bulk, equilibrium block polymers into thin membranes requires manipulating the self-assembled nanostructure away from equilibrium. The community is just beginning to understand this aspect of the block polymer membranes.”
One of the biggest issues in water treatment today is an inability to treat whatever comes down the pipe, so to speak.
“Yes, that is part of the vision for these materials as membranes for water treatment,” remarks Dr. Phillip. “The ability to build multiple functionalities into the membranes rationally and efficiently should prepare these materials to respond to and address whatever comes down the pipe.”
This kind of membrane may even have the ability to effectively filter pharmaceuticals out of wastewater—a major emerging problem in wastewater treatment.
“If the pore size of these materials can be pushed to the sub-nanometer range, these membranes should be able to better filter pharmaceuticals out of wastewater because of the high density of uniformly-sized pores they possess,” Dr. Phillip describes. “Additionally, at those small dimensions, chemical affinity plays an important role in the separation mechanisms. Therefore, we think the ability to manipulate the pore wall chemistry of the membranes offers new avenues to developing membranes that remove pharmaceuticals from wastewater through chemical affinity.”
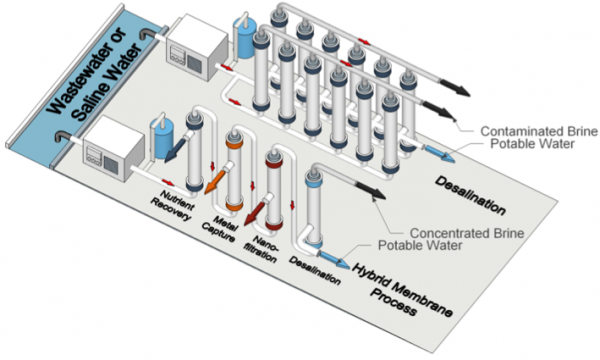
A conceptual process flow diagram for water treatment by a desalination process and a hybrid membrane process. In contrast to desalination, which focuses on producing potable water as the end product, the hybrid membrane process is designed with a cascade of separations that target the generation of product water at the purity required for the end applications as well as the recovery of valuable resources. In this type of process, resource recovery and selective solute removal can be achieved by membranes with functional pore wall chemistries, as represented by each module, that are integrated in series. Many material platforms are in development for deployment in these processes. Self-assembled block polymer membranes have great potential for implementation in desalination and hybrid membrane processes due to their well-defined nanostructure and nearly-limitless surface chemistries. Please note membrane modules are typically operated in a horizontal orientation; however, they are displayed vertically in the schematic to conserve space. (Credit: Zhang et al., via communication with Notre Dame press office)
Dr. Phillip’s team was able to create membranes with pore walls decorated with multifunctional chemistries that bind and sequester multiple contaminants—two at once in the study. In the future, this kind of membrane may be able to filter out multiple compounds. Moreover, the chemical composition of the block polymer matrix may be able to reduce issues with biofouling during desalination.
The well-defined nanostructure of the block polymers and the potential to introduce multiple functional chemistries within the pore walls of the membranes has the potential to make block polymer membranes more size-selective or chemically-selective,” clarifies Dr. Phillip. “However, the chemical composition of the block polymer matrix, which should be more resistant to low concentrations of oxidants such as chlorine, may help address issues with biofouling in desalination applications.”
Typically, prior to desalination, treated water is subjected to a de-chlorination step. This is essential, Dr. Phillip explains, because the performance of state-of-the-art desalination membranes declines irreversibly in the presence of chlorine, but without chlorine, the system is susceptible to biofouling. Therefore, eliminating the need to dechlorinate the water while maintaining a low concentration of chlorine during desalination may solve the biofouling problem.
The team predicts significant global applications for this technology, especially in places where water scarcity is high and resources are limited.
Top image: Dr. William Phillip. (Credit: Matt Cashore/University of Notre Dame, https://www.eurekalert.org/multimedia/pub/169728.php)











0 comments